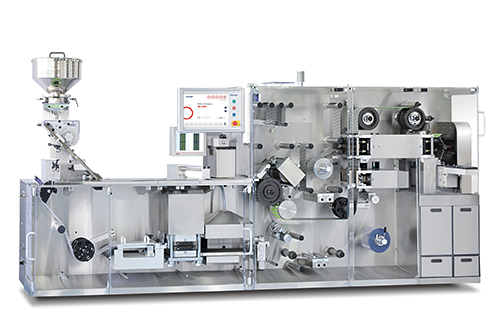
High speed blister machine HM400 P
Blister machine HM 400 P - High Output with Minimum Space, Continuous Motion, Drum Sealing. Balcony style designed and constructed in accordance with the requirement of the pharmaceutical industry and in observance of GMP standards. To meet pharmaceutical-complaint packaging concepts, production and mechanical drive systems are entirely separated in order to keep from the contamination of products. Both areas are easily accessible for maintenance and cleaning. This model is capable of max. 400 blisters per minute within a confined space, in continuous motion with drum sealing at dual lane system. By tool-less design of change part, it allows easy and fast tool change in less than 30 minutes. Latest technology of servo driving system secure the highest accuracy and the most precise operation penetrating all major stations as well as relieve the change over time by electrically saved product parameters, light weight of parts and fast replacement.PC base HMI system contributes to various kinds of control, easy monitoring and tracking for data and production, uploading the latest programming
High speed blister machine HM400 P on manufacturer site
InterPharmTechnology® - representative of HOONG-A in Russia and CIS area
https://blister.ift.ru/
http://www.ift.ru
Moscow, Godovikova st. 9
8 (495) 950-5665