Новое решение по этикетировке AP400 3T TE от IMA LIFE SENSITIVE
Different offers are available on the international market to align the pharmaceutical drug packaging lines to the new rising requirements in terms of track and tracing.
There is no one solution that will fit all legislative requirements or customer requests. The best approach is to be clear which markets are being supplied by which lines and therefore determine the minimum requirements for product compliance. Companies then decide which approach they wish to adopt, above the basic requirements of legislative compliance at a corporate, geographic, or site level.
It is therefore essential that pharmaceutical industries sit down with key packaging equipment suppliers to agree the best approach for a phased, long-term, cost-effective, and sustainable program to suit their needs.
To cope with the Track & Trace process, labeling machines can be equipped with the following components:
• ink-jet, thermal-transfer, laser and hot-foil overprinting units to print bar-codes alphanumeric
codes or 2D codes (Datamatrix);
• overprint control systems;
• camera systems to control OCV/OCR;
• 1D/2D or DM verification, presence, slope control, pattern matching, etc.
The offer may be completed with the possibility to apply and control RFID labels and integration with ePedigree and serialization systems. Even if many of these activities are not IMA LIFE’s core business, its awareness of the importance of having a comprehensive offer to meet mass serialization increasing needs, brought to a close cooperation with specialized companies who have knowledge in vision systems and management information systems. Ad-hoc solutions can be tailored to new packaging lines in order to meet with customer’s stringent and country specific requirements of the pharmaceutical industry.
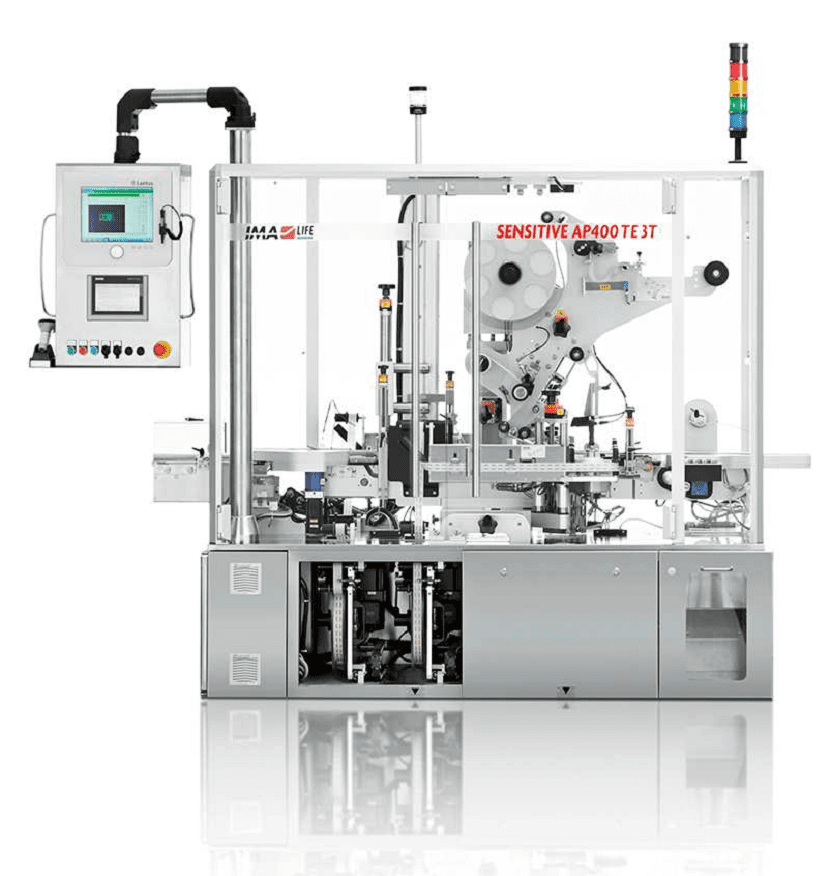
The SENSITIVE AP400 Series is a leading labeler on the market, since it can answer to the most different requirements in terms of track & trace, with a lot of machines already up and running worldwide.
The machine has been developed according to a modular constructive design and is available in different combinations with several applications to be customized according to the country legislation requirements and to the specific approach of the pharmaceutical industry.
IMA LIFE’s labeling equipment feature the application of labels on one, two or three faces of the carton (only on top or only on lateral sides or on all of them), thus providing a reliable method to securing against the threats of counterfeit, misbrand and adulterated drugs. The possibility to host up to 3 labelling heads and the high output (up to 450 cpm) gives the machine a high degree of flexibility.
The positive transport of cartons by means of toothed belt assures the indexing of each carton and avoids the possibility of misplaced ones or jams. As well, the correct inter-distance between cartons and labeling head is constantly guaranteed.
HOW TO ANSWER EMERGING SERIALIZATION REQUIREMENTS
There are a number of key trends in the pharmaceutical market causing the increase of the counterfeit problem. Massive growth of Internet sales and also unlicensed markets, as well as the huge potential for producing copy medicines, all help to flood the market with counterfeits.
Technology-based devices can be an efficient means to tackle counterfeit if a unique identification number for each individual pack is created and maintained throughout all stages of the supply chain. Two the strategies to face this threat: Mass Serialization and ePedigree.
Most of Pharmaceutical Companies identify Mass Serialization with DataMatrixECC200 codes (2D) as the best and easiest method which incorporates the main information to be traced in a much smaller space compared to the standard barcodes. The coding solution provides an efficient and cost-effective method to meet the EC’s requirements for pack identification put forth in the recently adopted Falsified Medicines Directive (July 2011).
In short European industry is calling for:
- A harmonized coding and serialization system across Europe for greater patient safety;
- A stakeholder governed point-of-dispense system for cost-effectiveness;
- A 2D data matrix based on international standard as unique identifier for interoperability.
According to the FDA Prescription Drug Marketing Act (2004) and the Florida Drug and Cosmetic Act (2005), any given prescription drug requires an electronic pedigree to accompany/validate drug distributions, in an attempt to prevent counterfeit developing standards for interoperability with unique identifiers.
 |
The e-Pedigree is a record in electronic form containing information regarding each transaction resulting in a change of ownership of the given prescription drug, including returns, thus to show the lineage of the drug from the manufacturer trough to the current point in the drug distribution channel (wholesaler, re-packager, pharmacy). In order to discourage companies from developing their own incompatible proprietary systems of ePedigrees, they have to be created and maintained in an interoperable electronic system, to be more precise an electronic track & trace system that uses a unique identification number, established at the point of manufacture, contained within a standardized non-proprietary data format and architecture and uniformly used. |
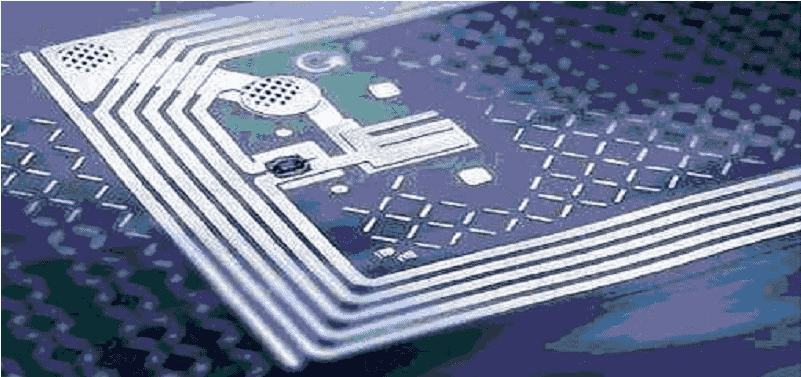
The unique serialized number will mostly likely be carried on either on a 2D bar code or an RFID chip placed on the saleable unit by the manufacturer and contains:
- prescription drug information;
- transaction and source information;
- ownership information;
- certification.
Anyway, RFID is unlikely to be implemented below pallet level.
11.10.2017





